Background IKZF1 deletion is frequently observed in Ph + acute lymphoblastic leukemia (Ph + ALL), which results in poor prognosis and affects clinical efficacy, and possible resistance to tyrosine kinase inhibitors (TKIs). Ikaros transcriptionally regulates the gene expression of multiple oncogenic pathways including Ras/MAPK signaling. However, it is undefined about the role of IKZF1 genetic defects in the activation of Ras/MAPK signaling and their relations with the efficacy of therapeutic drugs in ALL. This study is to investigate the sensitivity of IKZF1-deletion-mediated activation of RAS signaling to the new combined therapy of TKI Flumatinib (FLU) and the Azacitidine (AZA) in B-ALL cells.
Methods The patients' samples were obtained from Zhongda Hospital Southeast University with the approval of the institute's Ethics Committee. The gene expression was tested by qPCR or Western blot. CCK8 Cell proliferation, PI staining cell cycle analysis, and apoptosis assay (Annexin V-FITC/ PI staining) were performed in SUP-B15, the Ph + ALL cells with IKZF1 deletion treated with DMSO control, FLU only, AZA only, and FLU+AZA. The overlapped differential expression genes (DEGs)with the pathways (GO term, KEGG) analysis and gene interaction networks were achieved in the potential oncogenic genes in ALL through databases (OMIM, TTD, and GeneCards), and drug targets over that of SwissTargetPrediction, SuperPred, and SEA.
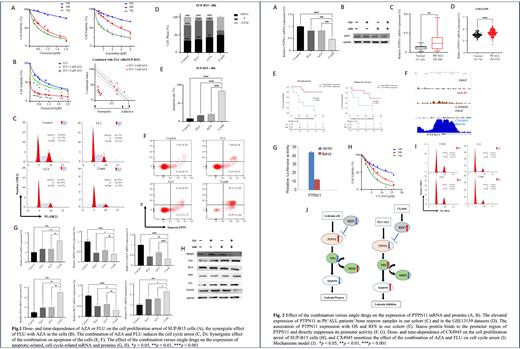
Results AZA or FLU treatment induced the cell proliferation arrest of SUP-B15 cells in a dose-/time-dependent manner (Fig. 1A). The combination of IC50 AZA with various doses of FLU significantly enhanced the effect on cell proliferation arrest versus single-drug controls, and CalcuSyn analysis demonstrated their synergistic effect in the cells (Fig. 1B). Also, the combination significantly increased the G0/G1 phase arrest (Fig. 1C, D), and % of apoptotic cells (Fig. 1E, F) versus the single-drug controls. Consistently, the combination up-regulated the mRNA and protein levels of P53, BAX, and P21, but decreased the MDM2, BCL2, and CyclinE (Fig. 1G, H). A total of 118 overlapped DEGs were identified between the potential oncogenes in ALL and FLU-/AZA-associated genes, and they predominantly enriched in Ras/MAPK oncogenic pathways, in which PTPN11 is at the top of the pathways. Indeed, the combination significantly decreased the mRNA and protein levels of PTPN11 (Fig. 2A, B). PTPN11 is significantly higher expressed in 20 newly-diagnosed Ph +ALL patients versus 20 healthy bone marrow control (Fig. 2C), and it was validated in the GSE13159 dataset (Fig. 2D). PTPN11 high expression is significantly associated with lower overall survival and relapse-free survival compared to that of low expression in our cohorts (Fig. 2E). These data together indicate the combination exerts anti-tumor effect by suppressing RAS/MAPK signaling through down-regulation of PTPN11, and also PTPN11 promotes Ras activation in Ph + B-ALL. Moreover, about 80% of Ph +B-ALL patients have the IKZF1 deletion, our ChIP-seq data showed that IKZF1-encoded Ikaros protein binds to the promoter region of PTPN11 and CK2 inhibitor CX4945 as Ikaros function activator dramatically increase the Ikaros binding to the promoter of PTPN11 in B-ALL cells and Ikaros directly suppresses its promoter activity (Fig. 2F, G). We have reported that CX4945 demonstrated therapeutic efficacy for high-risk B-ALL by restoring Ikaros' function. Here, we observed that pre-treatment of SUP-B15 cells significantly increases the effect of AZA+FLU combination on cell proliferation arrest, and cell cycle arrest (Fig. 2H, I). Taken together, these results reveal a model that IKZF1 deletion-mediated Ras/MAPK activation via PTPN11 has the oncogenic role in Ph + B-ALL and the combination of AZA+FLU exerts the anti-tumor effect by targeting Ikaros/PTPN11/Ras oncogenic signaling. The model is summarized in (Fig. 2J).
Conclusions The combination of AZA and FLU has a synergistic anti-leukemia effect on cell proliferation arrest and apoptosis in Ph +ALL cells with IKZF1-deletion by targeting Ikaros/PTPN11/Ras oncogenic signaling. Our data provide experimental evidence for a new potential combination of AZA with FLU in the therapy of Ph +ALL and highlight the likelihood of the novel combination in ALL patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal